PREN is an engineering calculation that helps us better understand how different materials are affected by pitting corrosion. Our engineering team has created this guide to cover PREN: from understanding pitting corrosion to using PREN equations to better evaluate your material’s corrosion risk factor.
 What Is Pitting Corrosion?
What Is Pitting Corrosion?
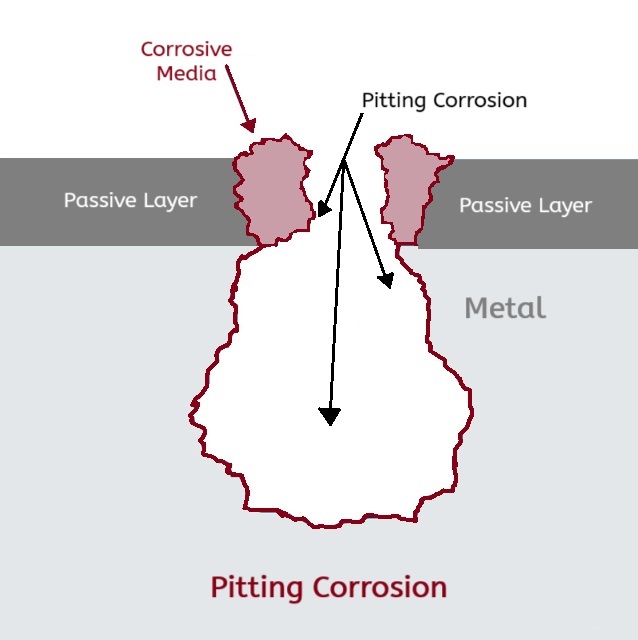
Before we delve into PREN, you need to first understand pitting corrosion. Pitting corrosion is a form of corrosion that causes small holes in metals and metal alloys. Pitting corrosion typically affects stainless steels when the chromium content is dissolved, leaving behind vulnerable iron. In common terms, you would know iron corrosion as rust.
Acid chlorides are the most common corrosive media – and cause for pitting – to stainless steel. When chlorides react with chromium in the passive layer, chromium chloride is produced with eats away the chromium in the passive layer. This damaging reaction creates iron chloride, which is now free to attack the base material at an alarming rate. Other corrosives that can cause pitting include:
• Bromides | • Fluorides | • Iodides |
Factors That Affect Pitting Corrosion
There are three factors that influence pitting corrosion:
• Chloride content
• pH
• Temperature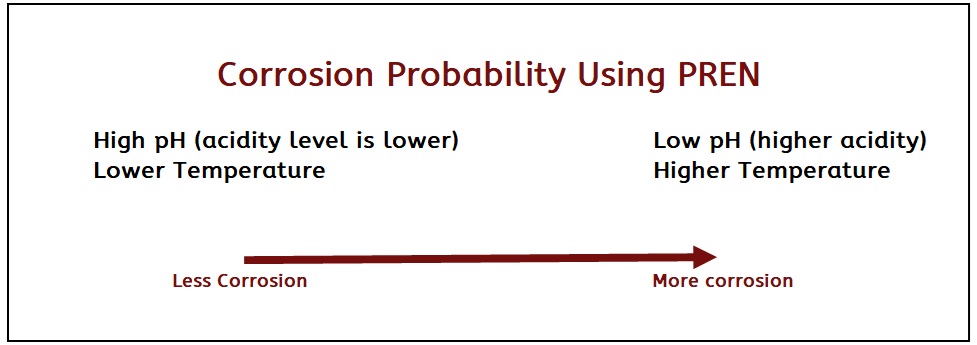 As a rule of thumb, the higher the temperature and chloride content and lower the pH, the greater probability of pitting corrosion. On the other hand, lower temps and a higher pH will reduce pitting risks. Please note, once pitting starts it worsens rapidly!
As a rule of thumb, the higher the temperature and chloride content and lower the pH, the greater probability of pitting corrosion. On the other hand, lower temps and a higher pH will reduce pitting risks. Please note, once pitting starts it worsens rapidly!
Preventing Pitting
One important factor to note is that the addition of molybdenum and/or nitrogen to stainless steels can improve their resistance to pitting corrosion.
 Time to Dig Into PREN: What It Is and How to Use It
Time to Dig Into PREN: What It Is and How to Use It
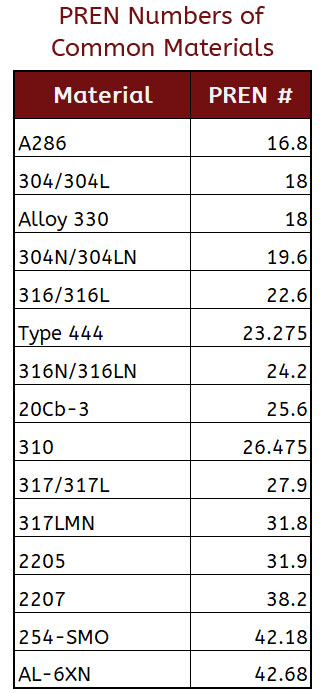
In our world of acronyms, PREN stands for Pitting Resistance Equivalent Numbers. Though it is not a 100% fool-proof way to calculate the potential for corrosion, it is a solid basis for comparing alloys and their resistance to pitting.
The specific chemical composition of the stainless-steel alloy determines a PREN number, based on the equations:

PREN numbers can help to determine if a certain stainless steel provides adequate pitting corrosion resistance for your application. For example, a PREN of 32 MIN is a good reference for oil and gas applications, where a PREN of 40 MIN could potentially be used for hydrogen sulfide or seawater applications.
- PREN numbers should be treated as a good reference point.
- PREN numbers can only be compared between stainless steels in the same family (ferritic, austenitic, duplex, etc.).
- A PREN of 32 is a common minimum for use in oil and gas industries.
- A PREN of 40 is a common minimum for use in hydrogen sulfide (H2S) environments, and seawater applications.
How Are Nickel Alloys Different?
Nickel alloys are recommended in highly aggressive environments due to their improved resistance to pitting compared to stainless steels. The main reason nickel alloys are so successful in corrosive environments is quite simple – Nickel. Nickel is an extremely corrosion resistant metal which replaces iron as the primary element in nickel alloys. While PREN is an excellent calculation for determining the corrosion resistance of stainless steels, it does not apply to nickel alloys or other corrosion resistant materials.
Our Engineers Are Here to Help
If you are working on an application that requires a high resistance to pitting corrosion, be sure to contact our materials engineers for material insight and recommendations.

